Phosphorus, having atomic number 15, has an electron composition of 2, 8, 5. Therefore, it has 5 electrons in its outermost shell. Chlorine has 7 electrons in its outermost shell, owing to its atomic number 17 and resultant placement 2,8,7. Step 2: To attain stability, each of the 5 Chlorine atoms will form a bond with Phosphorus. Phosphorus, however, has empty 3d atomic orbitals that can be used to expand the valence shell to hold 10 or more electrons. Thus, phosphorus can react with fluorine to form both PF 3 and PF 5. Phosphorus can even form the PF 6 - ion, in which there are 12 valence electrons on the central atom, as shown in the figure below. How many more electrons are there in the last energy level of phosphorus, atomic number 15, than there are in the last principal energy level of neon, atomic number 10?
How many valence electrons are in an atom of phosphorus?
1 Answer

Explanation:
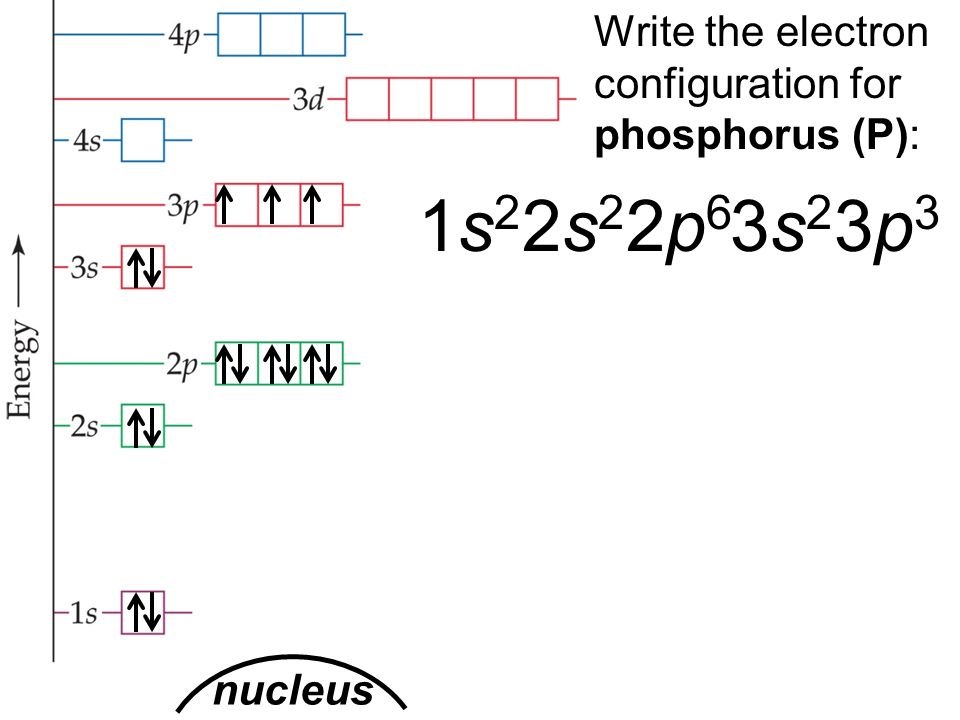
phosphorus has atomic number

it is in period
there are
Phosphorus Number Of Electrons Neutrons
there are
there are
this means that there are
all neutral atoms of the elements in group
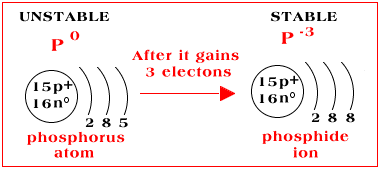
Phosphorus Number Of Electrons Gained Or Lost
Related questions